The Brains of Adult Stutterers: Are They Different From Nonstutterers?
 |
About the presenter: Janis Costello Ingham received her B.S. in Speech Pathology and Audiology from Northwestern University in 1964 and her master's and doctoral degrees in the same discipline from the University of Kansas in 1966 and 1969, respectively. Since that time she has served on the faculty of the Department of Speech and Hearing Sciences at the University of California, Santa Barbara. She holds the Credential of Clinical Competence in Speech-Language and is a Fellow of ASHA and a member of Division 4: Fluency and Fluency Disorders. Her research and teaching have focused on assessment and treatment of stuttering and phonologic disorders, particularly with children. Most recently she has concentrated her research efforts, with her husband, Roger, and colleague Peter Fox, on neurogenic and genetic aspects of stuttering. She has served as editor of the Journal of Speech and Hearing Disorders, as ASHA's Vice President for Research and Technology, and currently as Vice President for Research and Academic Development of the Council of Academic Programs in Communication Sciences and Disorders. |
 |
About the presenter: Roger Ingham is Professor of Speech and Hearing Sciences at the University of California, Santa Barbara. He was born in Australia and received his B.Sc. and Ph.D. in psychology (1972) from the University of New South Wales in Sydney, Australia. He has published 3 books and more than 150 papers, principally on developmental stuttering. His research has focused on the development of treatments and measures of stuttering, the neurology of stuttering and most recently its genetic basis. He is an ASHA Fellow and has been the recipient of several federally funded research grants for investigating stuttering. He also holds an adjunct appointment with the University of Texas Health Science Center, San Antonio, where, in collaboration with Peter Fox and his wife, Janis Costello Ingham, he has developed a program of research on stuttering using brain imaging techniques including PET, event-related fMRI and transcranial magnetic stimulation. |
The Brains of Adult Stutterers: Are They Different from Nonstutterers?
by Janis Costello Ingham and Roger J. Ingham
from California, USA
Introduction
The cause of stuttering has been a question of interest no doubt since the first person who stuttered spoke aloud to his or her first listener. Professor Bill Perkins, a well-known American scholar of stuttering, once pointed out that a hallmark of the field of stuttering is that no theory ever dies – they all live on forever. This could serve as a commentary on the fact that many theories of the cause and nature of stuttering have not been testable using the scientific method and so have not been able to be disproved – thus they linger on.
That something could be amiss in the neurologic systems of people who stutter - that is, in their brains, is one of the often-reappearing theories of the cause of stuttering. One particular area of interest has been the potential for problems in the auditory system of stutterers, especially given the fluency-inducing effects of auditory stimulation such as masking, chorus reading, and delayed auditory feedback.1 (The latter, of course, is now known to be more likely a by-product of slowed/prolonged speech rather than changes made to a speaker’s auditory feedback system.) Travis’s2 early theory of inadequate cerebral lateralization is another example. Although we know now that activity in both sides of the brain is intimately integrated and coordinated during speech production and perception, it is also well known that some degree of hemispheric specialization exists. In simplistic terms, for the vast majority of people (right handed and left handed) the left side of the brain is relatively more active during the processing of language; and the right side of the brain dominates when more global functions, such as spatial perception, are required.3 Variations on the theme of inadequate cerebral lateralization in people who stutter have suggested that the left hemisphere may compete with the right for the processing of language, or even that people who stutter may use exclusively the ill-equipped right side of their brains to process language. Until the last decade or so, support for such theories has been derived from inferential evidence. That is, scientists have had to rely on indirect measures of brain function such as electrical activity measured from the scalp during speech perception (not speech production, because movement artifacts interfere with the analysis of the signal),4 voice reaction times,5 dichotic listening,6 and finger tapping.7 Nonetheless, findings from many of these studies, especially those conducted through comparisons between performances of adult stutterers and nonstutterers, have offered support for the view that the brains of people who stutter function differently for speech production than the brains of those who do not stutter.
The development and increasing sophistication of various brain imaging technologies have further enabled researchers to study the brains of people who stutter. Now it is possible to observe brain activity more directly, in three dimensions throughout the entire brain, and even during actual speech. The bulk of the remainder of this paper will concentrate on presenting an overview of the “San Antonio studies,” – that is, one aspect of the brain imaging research that we have been conducting since the early ‘90s with our colleagues at the Research Imaging Center (RIC) of the University of Texas Health Science Center, San Antonio – led by Peter T. Fox, M.D. and a diverse core of researchers and technicians whose expertise in brain imaging techniques, including the analysis of images, has been pioneering. (We are zooming in on our own research because of space limitations and because, of course, it is the work we know best. Others are also conducting research in this arena and some representative publications are contained in the reading list at the end of this paper, for your reading pleasure.)
Positron Emission Tomography (PET)
We began our brain imaging research program by utilizing positron emission tomography (PET) to identify cerebral regions related to the occurrence of stuttering. This procedure is commonly referred to as functional brain imaging. In a typical PET study the research subject lays face-up on a flat platform with his or her head held firmly in place inside the PET camera, which encircles the head. It is necessary to prevent head movement because in each condition of an experiment multiple scans of the brain are acquired and these several “pictures” are averaged together to produce the findings for that condition. Immediately prior to each scan, the patient is injected (through a line into a vein in the arm) with a small dose of radioactive water (H215O). When the radioactive material reaches the brain (less than 15 seconds following injection) the PET camera registers relative cerebral blood flow (CBF) throughout the brain. Previous research has demonstrated that blood flow increases in regions of the brain that are relatively more active during a given task and decreases in those that are relatively less active during that task. In our research, each scan lasts 40 seconds and scans are taken about 10 minutes apart, which provides sufficient time between scans for the radioactive tracer to dissipate and wash through the person’s system. The findings of each subject’s scans are overlaid onto an anatomical MRI (magnetic resonance image) of that person’s brain and then those data sets are combined across subjects to produce a single data set averaged across all subjects in the group (e.g., stutterers or nonstutterers). The computer-processed data on which we rely are in two forms. One, we look at the overlays that display a group’s relative cerebral blood flow in different regions of the brain. These are the well-known colorful images of brain activity that indicate, by the use of a color scale, areas of the brain with relatively intense blood flow, usually colored bright red or yellow, through to regions of apparent deactivation (relative to the brain activity at rest), which are displayed in greens. When regional CBF is viewed in this manner, the images are typically presented in the horizontal view as a series of individual 4-6 mm thick “slices” of the brain (from the top of the head through to the brain stem, as though one were looking down on the brain from the top). (See the images presented on page 10.)
A more refined analysis of the images is obtained from computer-based statistical analyses of changes in the regional tissue uptake of the H215O. This is accomplished through “subtraction analyses” wherein CBF levels from scans obtained when the brain is at rest (subject lying still with eyes closed) are subtracted from CBF levels during activations generated by specific experimental conditions. These calculations produce a list of pinpointed locations in the brain where levels of CBF are statistically significantly higher or lower than the brain’s CBF at rest. These locations are described as clusters of voxels (voxel = 2x2x2 mm area) present at identified coordinates in Talairach space,8 where x = the right-left dimension, y = the anterior-posterior (front-back) dimension, and z = the superior-inferior (high-low) dimension. This “mapping” of locations in the brain activated (or deactivated) by particular, carefully selected tasks, is fundamental to functional brain imaging techniques and has led to now abundant information regarding regions of the brain associated with normally produced speech (mostly based on single word responses).9,10 For example, if one had a Talairach and Tournoux “map” handy, one could see that a cluster of voxels significantly activated at x = -46, y = -10, z = 40 by a group of control subjects reading aloud means that this spot in the precentral gyrus of the frontal lobe, which represents mouth movement, is activated by oral reading.
Given this somewhat over-simplified tutorial on PET brain imaging, let’s review the findings that this procedure has produced from our work with adult males and females who stutter. One of the early questions we asked was whether the brains of stutterers and nonstutterers are different when they are not speaking, that is, when their brains are “at rest.”11 We compared the resting-state CBF of 10 adult men who were chronic developmental stutterers and 19 age-matched men who did not stutter. All were right handed and all received 3 resting-state scans. Comparisons were made across 37 regions in each hemisphere, encompassing essentially the entire brain. No significant differences between stutterers and nonstutterers were found. This demonstrates that, in terms of CBF at least, there are no fundamental differences in stutterers’ and nonstutterers’ brains. If such differences exist, it seems they would have to be associated with how the brain functions during speech production.
Data regarding the latter hypothesis were obtained in another study.12 PET scans obtained from 10 male adult left handed stutterers were compared to scans from 10 age- and sex-matched left handed normally fluent controls. Subjects received 3 scans in each of 3 conditions: reading aloud (during which all of the stutterers stuttered), reading in chorus with another speaker (during which none of the stutterers stuttered), and eyes-closed rest (for subtraction purposes). Stuttering was associated with patterns of brain activity in particular regions that were highly different from controls, primarily in the motor system, as follows: M1 (brain region for mouth movements) was right- rather than left- lateralized; SMA (supplementary motor area, at the top of the brain) was more broadly and intensely activated; lateral Brodman Area (BA) 6 (superior lateral premotor cortex) was strongly activated on the right (and barely activated at all by the controls); and cerebellar activations more than doubled the extent observed in controls. Stutterers alone activated several regions: bilateral insula, and left claustrum, thalamus, and globus pallidus. Another important finding was that only stuttering was associated with deactivation in the auditory areas of the superior and posterior temporal lobe. When patterns of CBF during stuttering were compared with the fluency induced by chorus reading, brain activity of the stutterers approached the patterns observed in the controls, but the stutterers’ neural systems did not completely normalize.
As we all recognize, when people who stutter read or speak, not all syllables are stuttered. Therefore, in the study described above, the production of both stuttered and nonstuttered syllables occurred in the “stuttering condition;” therefore, the cerebral regions activated (or deactivated) during this condition were the product of both stuttered and nonstuttered behaviors. In order to refine the above analysis so that cerebral areas associated with stuttered and nonstuttered speech could be differentiated, a follow-up project13 using the data from the above-described study was conducted. A performance-correlation analysis was performed, based on the principle that the intensity of brain activations is highly correlated with the frequency of the behaviors they generate. In this case, regions of brain activation/deactivation that were correlated with the frequency of stuttering and the frequency of syllable production (essentially, fluent speech) were separately identified. This more sensitive analysis still turned up the same findings: that stuttering is associated with overactivity of the right cerebral regions associated primarily with speech planning and execution, and with underactivity of auditory regions within the temporal lobe. In addition, nonstuttered speech in these male stutterers is associated with left-sided overactivity in cerebellum. (Lateralization in cerebellum is typically opposite to that of the cerebrum).
A logical interpretation of the findings from these studies is that stuttering appears to stem from a neurologic system that has weaknesses in regard to (1) the selection and organization of the speech motor plan and (2) the use of the auditory system for self-monitoring of one’s speech.
One drawback of these studies is that the experimental design doesn’t allow one to determine if the aberrant areas of cerebral activity are responsible for the stuttered speech production or if they are a side effect of the extra and unusual motor behaviors that occur when a person is stuttering - the old “chicken and egg” problem. To investigate this issue we enlisted 4 stutterers and 4 controls who had participated in the previously described PET study.14 They were asked, in one condition, silently to read a passage and imagine that they were stuttering, and in another condition silently to read the passage while simultaneously hearing it read by another person (the chorus reading stimulus) and imagine that they were reading fluently. (A third condition was eyes-closed rest, for subtraction purposes.) For the most part, regional activations and deactivations associated with imagined stuttering overlapped those associated with actual stuttering, including deactivations in the auditory system. A prominent, and logical, exception was activation of M1 (mouth movement), which did not occur prominently in the imagine condition. These findings indicate that overt stuttering is not a prerequisite for the prominent regional activations and deactivations associated with stuttering. Thus these unusual activations must not be merely a byproduct of the movements of stuttering but a fundamental component of the creation of a moment of stuttering.
There’s one more PET study in our current arsenal that is of particular interest because it compares brain imaging findings between men and women stutterers (and controls).15 Little research aimed exclusively at female stutterers exists, and no other in brain imaging, although differences in brain structure and function between men and women in the general population have been documented.16,17 In this study we precisely replicated the oral reading-chorus reading paradigm and performance-correlation analysis described for our previous study with 10 female right handed developmental stutterers and a matched group of controls. Their findings were compared to the results for males reported above. Indeed, there were differences, and similarities, between male and female stutterers in terms of brain regions that were exclusively associated with the presence of stuttered speech. The similarities, which one might assume are the aberrant activations/deactivations critical to stuttering because they are present in both genders, are: anterior insula, which is highly active during stuttered speech (also shown by to be defective in adults with apraxia18); the auditory association area of the temporal lobe (BA 21/22), which is essentially inactive during stuttering and which we theorize plays a role in self-monitoring; and Broca’s area (BA 44/45), which was also deactivated during stuttering – an additional finding gleaned from the more sensitive analyses carried out with these data. Broca’s area is well known to participate in normal speech motor planning and production.19 Even in these three areas, however, the men and women stutterers differed: females showed bilateral activation/deactivation, while the males’ patterns were unilateral (right sided for anterior insula and the temporal lobe, left sided for Broca’s area). The images on the following page illustrate some of these findings. They are horizontal slices that cross the temporal lobe at about the level of the ears (z = 6).
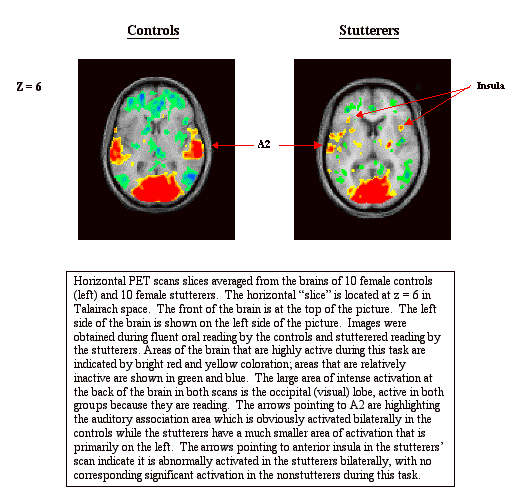
Gender-based differences for the females occurred primarily in activations during stuttering in the basal ganglia; for males, a major difference was seen in the prominence of cerebellar activations.
The exact meaning of all these findings continues to be illusive, especially because we do not yet completely understand the neural linkages among the areas that have been identified as playing a part in stuttering, or how these areas interact with cerebral regions that produce speech in general. From our findings we have learned, however, that the brains of people who stutter are indeed different from the brains of those who do not and that those differences appear to be confined to the functions of speech-motor planning (and, perhaps, production) and internalized auditory feedback. Whether these differences are present at the genesis of stuttering, or are the byproducts of our adult subjects’ years of stuttering practice, awaits imaging studies with children, which are on the horizon.
Some Words of Caution in Regard to PET Technology
Although PET methodology has offered landmark opportunities for researchers to observe the brain in action, there are certain drawbacks inherent in the current technology. First, we typically must study groups of patients, rather than individuals – although the design of more powerful PET cameras will soon alleviate this problem. Second, although PET has superior spatial resolution (that is, we can pretty accurately identify where in the brain activity associated with a given task is occurring), it does not offer sensitive temporal resolution. This is exemplified in our research wherein we study stuttered speech in 40-s periods of oral speaking that contain individual moments of stuttering embedded in sequences of nonstuttered syllables. (Debate exists, of course, regarding whether the “nonstuttered” syllables of stutterers are “normal” or also aberrant in ways that are not necessarily perceptually notable.20 In fact, some brain imaging studies of stutterers’ speech have not attempted to control for the presence or absence of stuttering.) Nonetheless, PET does not permit the analysis of a particular moment of speech, such as a moment of stuttering. Brain activity for the entire 40 seconds is imaged. Therefore, comparisons between stuttered and nonstuttered speech are less definitive because the stuttered sample also includes nonstuttered syllables (hence our use of performance-correlation analyses). We are currently doing pilot work with event-related fMRI that may solve this obstacle. Third, the ability of the subtraction design to segregate brain activity related exclusively to a particular behavior or cognitive activity has been questioned.21 A fourth, very serious shortcoming of the PET methodology is that it is not, indeed, a “standardized” methodology. Therefore, our ability to make meaningful comparisons among results of different brain imaging studies of stuttering is negatively impacted when studies differ in the (a) speaking/nonspeaking tasks imaged, (b) statistical analysis packages utilized, (c) brand of PET camera used, or particular PET technology used, and (d) subjects’ age, gender and perhaps severity of stuttering. Brain imaging research in stuttering is still a fresh, young area of research, and as more studies are conducted, results from disparate studies will begin to converge and pinpoint findings that consistently occur for stuttering. Some of that convergence occurs now, but one has to search for it!
Clinical Implications of Brain Imaging Findings
At this point in the short history of brain imaging research in stuttering, the clinical significance of the findings is untested. For many stutterers, certainly the knowledge that their stuttering most likely has a neurological foundation is of value. For some, it seems to confirm their personal experience with their disorder. In addition, the use of imaging to compare patterns of brain activity prior to and following successful treatment will inform clinicians and researchers about the kinds of changes that are necessary to produce and maintain fluency. (We have recently begun studies with untreated, recovered stutterers in order to gauge the nature of neurologic change necessary to produce fluency.) Imagining research will have clinical relevance if it eventually facilitates our capabilities in regard to recognizing consistent neural patterns that can differentiate between children whose stuttering will or will not persistent. And lastly and importantly, imaging might eventually be used to identify an individual’s stuttering speaker’s aberrant neural system(s) and thereby the clinician in tailoring treatment to modify that individual’s aberrant system. So, although scientists are still at the early stages of basic imaging research in stuttering, the clinical potential seems immense.
References
- Bloodstein, O. (1995). A handbook on stuttering. San Diego: Singular Publishing Company.
- Travis, L.E. (1978). The cerebral dominance theory of stuttering: 1931-1978. Journal of Speech and Hearing Disorders, 43, 278-281.
- Segalowitz, S., & Gruber, F. (Eds.) (1977). Language development and neurological theory. New York: Academic Press.
- Moore, W.H., Jr., & Haynes, W.O. (1980). Alpha hemispheric asymmetry and stuttering: Some support for a segmentation dysfunction hypothesis. Journal of Speech and Hearing Research, 23, 229-247.
- Adams, M. R., & Hayden, P. (1976). The ability of stutterers and nonstutterers to initiate and terminate phonation during production of an isolated vowel. Journal of Speech and Hearing Research, 19, 290‑296.
- Curry, F.W., & Gregory, H.H. (1969). The performance of stutterers on dichotic listening tasks thought to reflect cerebral dominance. Journal of Speech and Hearing Research, 12, 73-82.
- Webster, W.G. (1993). Hurried hands and tangled tongues. In E. Boberg (Ed.) Neuropsychology of stuttering (pp. 73-127). Edmonton: University of Alberta Press.
- Talairach, J., & Tournoux, P. (1988). Co-planar stereotaxic atlas of the huma brain. Verlag” Thieme Medical Publishers.
- Fiez, J.A., & Petersen, S.E. (1998). Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences, USA, 95, 914-921.
- Indefrey, P., & Levelt, W.J.M. (2000). The neural correlates of language production. In M.S. Gazzaniga (Ed.), The new cognitive neurosciences (2nd ed) (pp. 845-866). Cambridge, Massachusetts: The MIT Press.
- Ingham, R.J., Fox, P.T., Ingham, J.C., Zamarripa, F., Martin, C., Jerebek, P., & Cotton, J. (1996). Functional-lesion investigation of developmental stuttering with Positron Emission Tomography. Journal of Speech and Hearing Research, 39, 1208-1227.
- Fox, P.T., Ingham, R.J., Ingham, J.C., Hirsch, T.B., Downs, J.H., Martin, C., Jarabek, P., Glass, T., & Lancaster, J.L. (1996). A PET study of the neural systems of stuttering. Nature, 382, July 11, 158-162.
- Fox, P.T., Ingham, R.J., Ingham, J.C., Zamarripa, F., Xiong, J.-H., & Lancaster, J. (2000). Brain correlates of stuttering and syllable production: a PET performance-correlation analysis. Brain, 123, 19885-2004.
- Ingham, R.J., Fox, P.T., Ingham, J.C., Zamarripa, F. (2000). Is overt stuttered speech a prerequisite for the neural activations associated with chronic developmental stuttering? Brain and Language, 75, 163-194.
- Ingham, R.J., Fox, P.T., Ingham, J.C., Zamarripa, F., Xiong, J.-H., Hardiews, L.J., & Lancaster, J.L. (in preparation). Brain correlates of stuttering and syllable production: A gender replication.
- Shaywitz, B.A., Shaywitz, S.E., Pugh, K.R., Constable, R.T., Skudlarski, P., Fulbright, R.K., et al. (1995). Sex differences in the functional organization of the brain for language. Nature, 373,607-609.
- Pugh, K.R., Shaywitz, B.A., Shaywitz, S.E., Constable, R.T., Skudlarski, P., Fulbirght, R.K., et al. (1996). Cerebral organization of component processes in reading. Brain, 119, 1221-1238.
- Dronkers, N.F. (1996). A new brain region for coordinating speech articulation. Nature, 384, 159-61.
- Fox, P.T. (1995). Broca’s area: Motor encoding in somatic space. Behavioral and Brain Research, 18, 344-345.
- Smith, A. (1999). Stuttering: A unified approach to a multifactorial, dynamic disorder. In N. Ratner & E.C. Healey (Eds.). Stuttering research and practice: Bridging the gap (pp. 27-44). Mahwah, NJ: Lawrence Erlbaum.
- Ingham, R.J. (in press). Neuroimaging and developmental stuttering: Emerging controversies, findings and directions, Or, On avoiding petard hoisting in Athen, Georgia. In A.K. Bothe (Ed.). Evidence-based treatment of stuttering: Empirical issues and clinical implications. Mahwah, NJ: Lawrence Erlbaum Associates.
Additional Readings on Brain Imaging and Stuttering
- Braun, A. R., Varga, M., Stager, S., Schulz, G., Selbie, S., Maisog, J. M., Carson, R.E., & Ludlow, C. L. (1997). Altered patterns of cerebral activity during speech and language production in developmental stuttering. An H215O positron emission tomography study. Brain, 120, 761-784.
- De Nil, L.F., Kroll, R.M., & Houle, S. (2001). Functional neuroimaging of cerebellar activation during single word reading and verb generation in stuttering and nonstuttering adults. Neuroscience Letters, 302, 77-80.
- De Nil, L.F., Kroll, R.M., Kapur, S., & Houle, S. (2000). A positron emission tomography study of silent and oral single word reading in stuttering and nonstuttering adults. Journal of Speech, Language, and Hearing Research, 43, 1038-1053.
- Finitzo, T., Pool, K. D., Freeman, F. J. Devous, M.D., & Watson, B. C. (1991). Cortical dysfunction in developmental stutterers. In H. F. M. Peters, W. Hulstijn, & C. W. Starkweather (Eds.) Speech motor control and stuttering. (pp 251-261). Amsterdam: Excerpta Medica.
- Foundas, A.L., Bollich, A.M., Corey, D.M., Hurley, M, & Heilman, K.M. (2001). Anomalous anatomy of speech-language areas in adults with persistent developmental stuttering. Neurology 57, 207-215.
- Ludlow, C. L. (2000). Stuttering: Dysfunction in a complex and dynamic system. Brain, 123, 1983-1984.
- Pool, K. D., Devous, M. D., Freeman, F. J., Watson, B. C., & Finitzo, T. (1991).
Regional CBF in developmental stutterers. Archives of Neurology, 48, 509-512. - Salmelin, R., Schnitzler, A., Schmitz, F., & Freund, H.J. (2000). Single word reading in developmental stutterers and fluent speakers. Brain, 123, 1184-1202.
- Salmelin, R., Schnitzler, A., Schmitz, F., Jäncke, L., Witte, O.W., & Freund, H.J. (1998). Functional organization of the auditory cortex is different in stutterers and fluent speakers. Neuroreport, 9, 2225-2229.
- Sommer, M., Koch, M.A., Paulus, W., Weiller, C., & Büchel, C. (2002). Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet, 360, 380-383.
- Wood, F., Stump, D., McKeehan, A., Sheldon, S., & Proctor, J. (1980). Patterns of regional cerebral blood flow during attempted reading aloud by stutterers both on and off haloperidol medication: Evidence for inadequate left frontal activation during stuttering. Brain and Language, 9, 141-144.
- Wu, J.C., Maguire, G., Riley, G., Fallon, J., LaCase, L., Chin, S., Klein, E., Tang, C., Cadwell, S., & Lottenberg, S. (1995). A positron emission tomography (F-18) deoxyglucose study of developmental stuttering. Neuroreport, 6, 501-505.
September 2, 2002

